A “review” of the Chemistry SeedKit (Lab-in-a-Box)
The teachers were like giddy teenagers who just laid hands on the newest gadget. They were smiling from ear to ear, posing, taking photos of each other. What caused such excitement?
The marvel that was in their hands: a colorful balloon inflated by a reaction between eggshells and vinegar. Remember the joy and wonder when YOU first did it?
***
We were excited to test several activities created by SeedKit. SeedKit stands for “Science Education Equity Development Kit“, and is a collaborative of like-minded folks based at Wellesley College. The group was started by the amazing Kayla Bercu, a soon-to-be senior who, since high school, had been working with the SEGA School of Secondary Education for bright but impoverished girls in Morogoro, Tanzania.
Based on her observations in the classroom and discussion with teachers there, Kayla has been working with her fellow students at Wellesley to develop a set of experiments that can be packaged and used for hands-on instruction in low-resource countries.
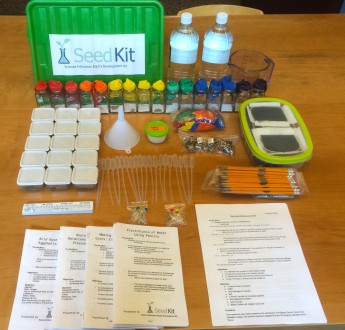
The SeedKit is a actual lab-in-a-box, designed for senior secondary school, with proper pre-labs, worksheet and a post-lab evaluation.
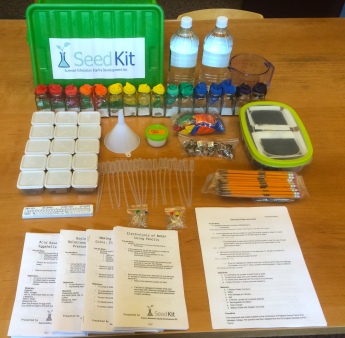
Chemistry Kit. Credit: SeedKit
For this training, we tested the brand new chemistry experiments, adapting the activities and concepts for our after school clubs, and for teachers who work in p2 to p7 (JHS2), a wide range of expertise and knowledge.
Below, we “rate” the few activities that we shared with the teachers, based on the ability to delight and engage (wow factor), how adaptable the activity is for explaining related concepts for younger students (adaptability), how conducive the activity is to further investigation and invites questions, especially if presented before the “theory” (inquiry) and if the materials in the kit might be obtained locally or substituted with locally available materials (materials).
1) Eggshell and Vinegar Reaction
A simple variation on the baking soda and vinegar experiment to demonstrate chemical change.
Wow factor: 5
As mentioned the teachers were charmed by the inflated balloons.
Adaptability: 4
Even without understanding the detailed chemistry and stoichiometry, teachers and students can appreciate the phenomenon of combining two reagents – one solid, one liquid – to produce a different substance – gas.
Inquiry: 5
In addition to the suggested follow-up to measure the volume of gas produced, teachers decided that students can (visually) compare the difference in volume of gas produced
a) by varying the amount of vinegar added, or
b) as a function of the surface area/size of the broken pieces, if each group started with the equivalent of one egg shell.
- A few who didn’t read the instructions sheet wondered what the gas was – some wondered if it was flammable. Since I knew it was CO2, they tested the gas by releasing it over a flame! This gave us a chance to discuss safety procedures.
- We wondered but didn’t have time to try if the addition of a pH indicator, will allow a visual progress of the reaction to be followed as the amount of vinegar decreases.
Materials: 4
- The glass jars can be substituted with other similar containers.
- Vinegar is cheap and readily available.
- Large amounts of eggshell can be obtained from street vendors who sells hard boiled eggs (yum).
- Balloons might be substituted by polyethylene bags (though we do care about the environmental impact of both). In either case, both can be re-used.
2) Wet cell / chemical battery to power an LED light
This is a wonderful way to demystify what’s in a “dry cell”.
Wow factor: 4
Truthfully, anything that can light up an LED is a win! =-)
Inquiry: 4
- The teachers wondered whether citrus fruit juices might be used.
- They discussed using water, and water with salt as comparison.
Adaptability: 3
Upper primary and JHS students should be able to grasp concepts related to circuits, electricity and electrolyte solutions. Light emitting spectrum may be more difficult to understand. The investigative activities listed above are certainly doable for .
Materials availability: 5
- Vinegar and cardboard are readily available
- We wondered if any of the Ghanaian coins can be substituted for the US pennies provided. Need to do some research/testing!
3) Electrolysis
A simple and affordable way (OK, maybe except the batteries) to visualize this reaction, which typically involves expensive equipment.
Wow factor: 5
The teachers loved the easily discernible bubbles that emerged.
The SeedKit suggested a pH indicator. Teachers were delighted to know that they can use hibiscus to make a pH indicator (red drops below) and that it changed colors at one of the electrodes!
Adaptability for younger grades: 3
- Students can visually estimate the differential production of bubbles at each electrode.
- The chemical reaction can be taught to the JHS students – one JHS teacher definitely wrote out the equation “spontaneously”.
Inquiry: 5
Teachers were very curious about the gas produced – volumetrically and the chemical nature. (Again, we didn’t do a pre-lab). As the SeedKit and post suggested, we were able to determine which electrode produced hydrogen based on the color change of the indicator. Teachers were also able to confirm this by thinking about the ratio of hydrogen to oxygen in a water molecule and their composition as gases.
Materials availability: 4
The pencils are great but might consider using the electrodes from a dry cell (to be removed carefully by a teacher or supervised student). For older students this may be
The use of rechargeable batteries and affordable DIY solar panels are laudable and should be pursued. The rechargeable batteries would be an investment but be economical in the long run and better for the environment.
***
In all, we give the Chemistry SeedKit a solid 4, which is high marks since it wasn’t originally developed for the age group that our teachers would be addressing. Science is awesome because the same reaction / activity has multiple ways to engage and for learning to happen, from observing cool phenomenon, to simple investigations, to big ideas and concepts, to specific details.
We are so grateful to have partnered with SeedKit this summer and hope for many more opportunities to come!!